On September 6, 2023, the Luo Min/Lu Zhigang/Gao Hai collaboration team from the Institute of Biomedical Sciences of Fudan University and the Zhao Yun team of the Center for Excellence in Molecular Cell Science of the Chinese Academy of Sciences published a research article entitled CD300ld on neutrophils is required for tumour-driven immune suppression online in the journal Nature.
The study found a new, highly conserved tumor immunosuppressive receptor, CD300ld. CD300ld is highly specific for PMN-MDSCs and is a key receptor for regulating PMN-MDSCs recruitment and immunosuppressive function. Targeting CD300ld can reshape the tumor immune microenvironment by inhibiting the recruitment and function of PMN-MDSCs, thereby producing a broad-spectrum anti-tumor effect. CD300ld target shows good safety, conservation, anti-tumor efficacy, and synergy with PD1 target, which is expected to become a new ideal target for tumor immunotherapy.

Immune checkpoint blockade (ICB) therapy represented by molecules such as PD-1/PD-L1/CTLA-4 is a revolutionary progress in the field of tumor treatment in recent years. However, the effectiveness of existing ICBs varies greatly among patients with different tumors, with a significant proportion of patients not responding and a lower proportion of long-term benefit. The tumor immune microenvironment contains a large population of immune-suppressing myeloid cells, which plays a key role in tumor development and treatment tolerance, and is a serious hindrance to existing immunotherapy.
Pathologically activated neutrophils (also known as polymorphonuclear myeloid derived suppressor cells, PMN-MDSCs) are important components of the immunosuppressive microenvironment. Unlike normal neutrophils, PMN-MDSCs have a strong inhibitory effect on lymphocyte killing and are involved in tumor progression through multiple pathways. Finding specific targets for PMN-MDSCs to regulate the tumor immune microenvironment is the focus and challenge of current immunotherapy.
As the body's first line of defense, neutrophils play an important role in innate immunity, but due to the short half-life of neutrophils themselves and their inability to continue to proliferate after maturity, research on such cells lags behind other innate and adaptive immune cells.
With the advancement of technology in recent years, the complexity and heterogeneity of neutrophils in physiological and pathological states have begun to be understood, and their role in cancer has attracted more and more attention. Many clinical evidence also shows that neutrophils are deeply involved in tumor progression and are significantly related to patient prognosis, so it is of great clinical significance to explore the role of neutrophils in tumor progression and find specific targets for this group of cells.
Focusing on this scientific question, the researchers first targeted a series of membrane proteins biased by myeloid cells, and used mouse tumor models to screen CRISPR-Cas9 in vivo, and found that immune cells lacking CD300ld were the most significant deletion in tumors, suggesting that cells expressing CD300ld may promote tumor development. CD300ld was highly specific in neutrophils and significantly upregulated after tumor-bearing. Subsequently, the researchers constructed CD300ld knockout mice and confirmed that CD300ld deletion significantly inhibited the development of multiple secondary and primary tumor models. At the same time, CD300ld knockout had no significant effect on the normal development of mice and the development of immune system.
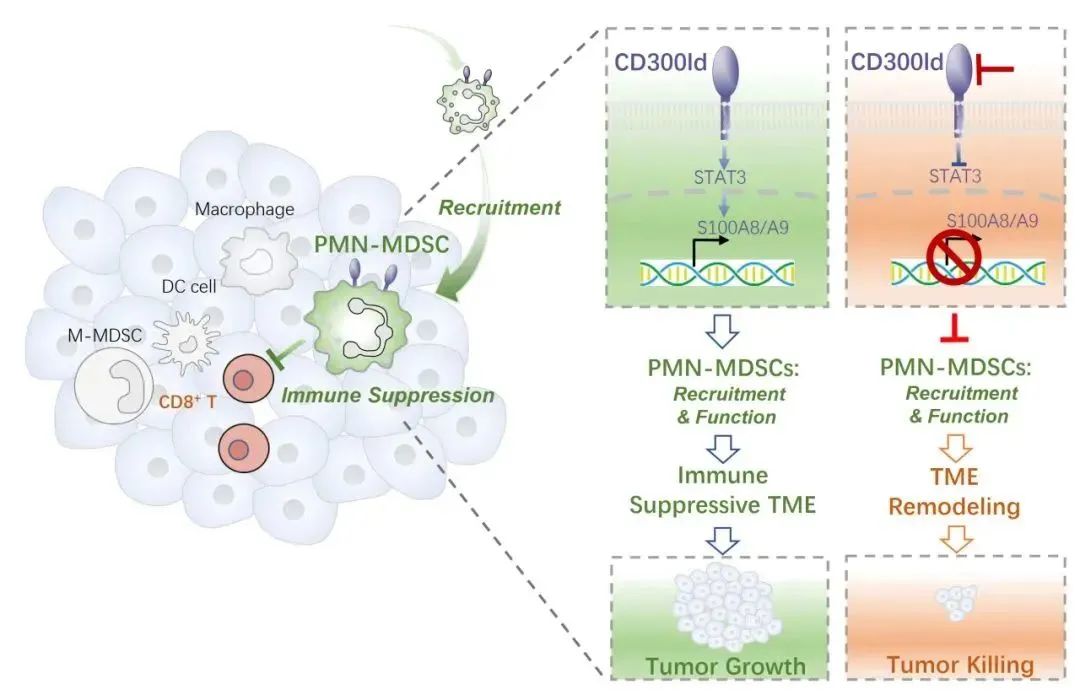
Through bone marrow transplantation, construction of a variety of conditional knockout mice, and removal of PMN-MDSCs in vivo, CD300ld was confirmed to regulate tumor development through PMN-MDSCs. Therefore, CD300ld, as a surface marker of neutrophils/PMN-MDSCs, is a key functional receptor for PMN-MDSCs to promote tumor development.
In order to study the mechanism of CD300ld regulating tumor immunity, the researchers first analyzed the effect of CD300ld deletion on the tumor microenvironment. CD300ld deletion led to a significant decrease in the recruitment of PMN-MDSCs in tumors, accompanied by a significant increase in CD8+ T cell infiltration. Single-cell transcriptome analysis showed that most of the tumor-promoting cell populations were significantly downregulated in proportion and inhibitory immune signaling pathway, while the anti-tumor cell population was significantly increased in proportion and corresponding signaling pathway. Therefore, CD300ld deletion can reshape the tumor immune microenvironment, transforming it from an immunosuppressive state to an immunoactivated state. This further reflects the key role of PMN-MDSCs in the establishment of tumor immune microenvironment.
Two properties of PMN-MDSCs are key factors in promoting tumor progression: their ability to recruit from tumors, and the ability to highly suppress immune effector cells. CD300ld regulates both functions of PMN-MDSCs. CD300ld-deficient PMN-MDSCs showed weaker migration and ability to recruit into tumors; At the same time, its ability to inhibit the proliferation of T cells is also significantly reduced. Further analysis showed that S100A8/A9 is a key effector molecule downstream of CD300ld. CD300ld regulates the expression of S100A8/A9 by activating STAT3 and upregulating the expression of S100A8/A9 to form the CD300ld-STAT3-S100A8/A9 axis, thereby regulating the recruitment and immunosuppressive function of PMN-MDSCs.
The researchers then explored whether blocking CD300ld after tumor formation still had anti-tumor effects. The results showed that after the tumor was built, the drug conditional knockout of CD300ld could still significantly inhibit the tumor development. The researchers further competitively inhibited CD300ld by injecting CD300ld extracellular protein, which was also able to significantly inhibit the progression of various secondary and primary tumors. Blocking CD300ld with PD-1 antibodies yields a better combination therapeutic effect. Therefore, blocking CD300ld shows good anti-tumor therapeutic potential and can produce significant synergy with existing ICB therapy.
To further investigate the synergy of human CD300ld, the researchers analyzed the TCGA database and multiple patient samples from different types of tumors. The results showed that CD300ld was also highly expressed on human neutrophils/PMN-MDSCs. At the same time, the expression of CD300LD was significantly higher than that of adjacent cancers and normal tissues in a variety of cancers, and was significantly related to the invasion of neutrophils and the prognosis of patients. The researchers then constructed CD300LD humanized mice, further demonstrating that human mouse CD300ld is functionally homologous, and competitive inhibition of human CD300LD also showed significant anti-tumor effects.
In summary, the study found that CD300ld, a key functional receptor on the surface of PMN-MDSCs, plays an important role in tumor immunosuppression. CD300ld simultaneously regulates the recruitment of PMN-MDSCs and their T cell inhibitory function through the STAT3-S100A8/A9 axis, thereby promoting the establishment of immunosuppressive microenvironment and tumor progression. Blocking CD300ld produces broad-spectrum anti-tumor effects by reducing the recruitment of PMN-MDSCs and reducing their immunosuppressive function, and reshaping the immune microenvironment from immunosuppressive state to activated state.
Wang Chaoxiong, postdoctoral fellow of the Center of Excellence in Molecular Cell Science, Chinese Academy of Sciences, Zheng Xichen, postdoctoral fellow of the Institute of Biomedical Sciences/Children's Hospital of Fudan University, and Jinlan Zhang, research assistant of the Institute of Biomedical Sciences/Fifth People's Hospital of Fudan University, are co-first authors of this paper. Researcher Luo Min, Institute of Biomedical Sciences/Affiliated Pediatrics Hospital of Fudan University, Researcher Yun Zhao of the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Researcher Lu Zhigang, Institute of Biomedical Research/Fifth People's Hospital of Fudan University, and Hai Gao, Institute of Biomedical Sciences/Xuhui Central Hospital of Fudan University, are co-corresponding authors of the paper.
Original link: https://www.nature.com/articles/s41586-023-06511-9
Source: Fudan University, Bioon Cells







 备案号:沪ICP备20003873号-1
备案号:沪ICP备20003873号-1

